
Celebrating the Road to Recovery
There’s a photo we’ve probably all seen, of a senior care employee with her arms in the air, jubilant and celebrating the vaccine she received just moments before. Nothing captures better all the emotions of that day, when hope was finally on the horizon and our vaccine clinics offered a huge step on the Road to Recovery. But getting to that moment meant overcoming countless hurdles, many that even seemed impossible, and was the culmination of the contributions and sacrifices of every employee. This is the story.
MobilizationFrom cold storage and software to daunting travel logistics, success was in the details. |
Ready, Set, Go-Time!The vaccine arrives—now it’s up to us to distribute it safely and in record time. |
Ready, Set, Go-Time!The vaccine arrives—now it’s up to us to distribute it safely and in record time. |
The CampaignUsing science and social media, we fought vaccine hesitancy and drove record participation. |
The CampaignUsing science and social media, we fought vaccine hesitancy and drove record participation. |
The GrindWith planes, trains and automobiles—and a fleet of RVs, vaccine distribution hit full speed. |
Finish LineWith the clinics completed, the results are in—55,000 vaccines delivered and countless lives saved. |
Looking aheadNow begins the final phase—a vaccine continuity effort to protect new residents and staff. |
The Decision
In a deadly pandemic crisis unlike anything the world has ever known, vaccines were the ultimate hope—and challenge. The logistics of distribution would be overwhelming, but early on we chose to aggressively pursue CDC selection as an approved vaccine provider—one of only seven long-term care pharmacies in the country to win that distinction. We knew it was going to be an incredibly expensive proposition, not only in dollars but in vast expenditures of time and energy. But our customers and those in their care deserved our best efforts and resources, and we did it for them—because it was the right thing to do.

Mobilization
The challenges were endless and formidable. A fragile vaccine that required sub-zero storage temperatures. Software to track consents, vaccinations and second shots. Communication systems, training and the herculean task of staffing mobile vaccination teams and getting them to hundreds of sites. But over endless meetings and strategy sessions, a battle plan took shape. We invested in three ultra-cold freezers and created a vaccination tracking application from scratch. We obtained consents in advance from large numbers of residents and staff. We advocated with governors and regulators that long-term care pharmacies were the best choice to deliver the vaccines to clients and staff. We showed leadership when others seemed paralyzed in inaction by the sheer scope of the task, and prepared for mobilization on a scale we had never attempted before.

Ready, Set, Go-Time!
It was one of those unforgettable days when you realize the world might never be the same, and that you’re part of something big. The vaccine arrived in the hands of an emotional Fed Ex driver—just a simple white box, but representing an historic moment on the Road to Recovery. Every next step, from just opening the box to preparing vials for delivery, required precise process discipline and pharmacy expertise, and pharmacists and their mobile teams practiced like they were preparing for a rocket launch. As some pharmacy consultants were asked to hit the road with mobile delivery teams, others picked up the slack in customer facilities, sometimes in entirely different states from their usual assignments. The successful mobilization required every employee to be ready and willing to do anything necessary to aid the vaccination effort.
First Shots
Our pilot vaccine clinic opened its doors on Dec. 21, 2020, one of the first in Oregon to do so, and fully a week before the national retail pharmacies were ready to mobilize. It was only fitting that our first resident to be vaccinated, and one of the first in Oregon and the nation, was a World War II veteran who had been decorated for bravery during the attack at Pearl Harbor. This war was against a far different enemy, but he was no less heroic, showing no fear as the needle plunged into his arm. With a wall of reporters and TV cameras documenting the moment, he encouraged others to step up and get the shot.
It was an emotional moment, one that would be repeated over the next three months in hundreds of Consonus senior care client communities in three states. After nearly a year of the pandemic’s daily and deadly toll, the vaccine revealed a glorious light at the end of the tunnel, and the first true hope in a long time.
The Campaign
Distributing the life-saving vaccines was just half the battle—the rest was convincing residents and staff it was safe and essential to take them. We quickly learned the media was causing great confusion, and while resident participation rates were extremely high, staff rates initially hovered just above 60 percent. To address fears and build momentum, we provided unbiased, science-based educational materials and videos that focused honestly on the facts. We launched a #GetVaccinated campaign on social media, resulting in 54 posts over 8 channels in 16 days. By the end of the second clinic, staff participation rates had jumped to 83 percent and positive case rates plummeted 53 percent. We also aggressively advocated for our Independent Living residents to receive the vaccine, and hosted our first clinic for that population on Dec. 31, 2020. It was truly a New Year’s Eve celebration we’ll never forget.
The Grind
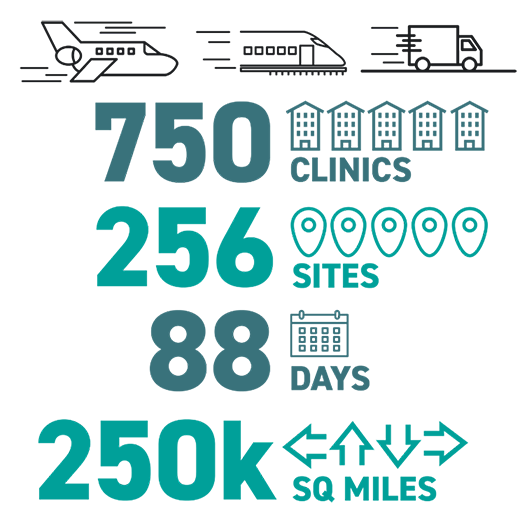
The decision to fight for vaccine distribution was the right thing to do, but the right thing isn’t always easy. Getting those precious vials where they needed to go required planes, trains, automobiles and RVs, and the challenges we faced included snow and ice storms, power outages and vehicle breakdowns. The RV logistics alone required the full-time attention of many of our team members, from big tasks like managing schedules to less glamorous essentials like waste disposal. From the Pacific coast, through mountain passes and out to the eastern edges of Oregon and Washington, we hosted 750 clinics at 256 sites, and covered 250 thousand square miles in 88 days. From Dec. 21, 2020 to Feb. 28, 2021, only five days were without clinics, and at one point we had hosted an average of 12 clinics per day for 40 consecutive days.
This massive effort would not have been possible without the dedicated staff and volunteers who knew there was a job to be done and lives to be saved. Pharmacy staff not directly involved in clinics helped keep medication deliveries on track, fulfilling a 98.5% same-day fill rate. Those employed in other divisions of Marquis and Consonus accepted an array of additional responsibilities on top of their regular day jobs. For everyone involved, the impact of their contributions was made clear in the daily, first-hand reports from those on the road of inspiring experiences at senior care facilities.
Finish Line
The Road to Recovery vaccination program featured three clinic cycles. These were designed to allow individuals who missed the first clinic to participate in the second, and to receive their second dose in clinic three. This included new staff and admissions, or individuals who had taken a wait-and-see approach. It was with a feeling of exhilaration and accomplishment that we crossed the three-clinic finish line on March 19, 2021. In just three months, we administered 55,070 vaccines, fully protected 27,535 individuals, achieved a 90%+ percent reduction in positive cases and saved countless lives. Through the dedication of our hard-working staff, the clinics succeeded beyond our wildest dreams. And yet, though the end of the pandemic is in sight, the work isn’t quite done, and we’re marshalling our efforts and energies to confront the final test.

Looking Ahead
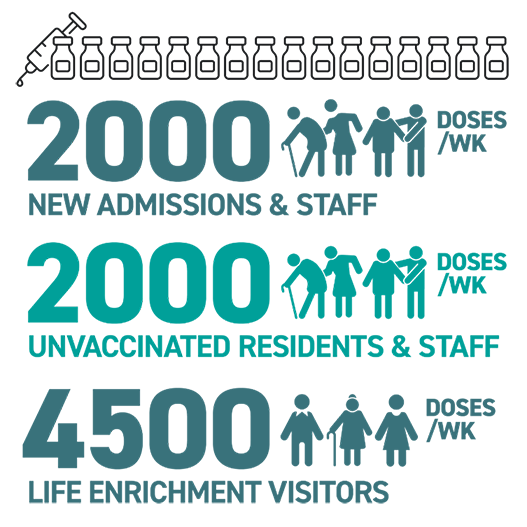
Though tremendously proud of our clinic achievements, we’re looking ahead to the next urgent challenge. New staff hires and resident admissions will continue to enter our senior care communities, and may not be fully vaccinated. As visitation restrictions are lifted, facilities will be monitored by their vaccine participation rate, making it critical that we continue to provide vaccines. There will even be opportunities to identify life enrichment visitors who may qualify for a vaccine in order to help reduce resident isolation with in-person visits. Though we’re now moving away from the clinic model, our vaccine continuity plan will allow us to dispense vaccines to our clients on an on-going basis. It may represent the final mile on the Road to Recovery, but it’s as important as any on the journey.
Our thank you extends to all who participated in this effort, including our customers, business partners, and health authorities. Nothing we’ve achieved would have been possible without the selfless contributions of each and every one of you. Thank you!
Mobile Team
Oregon
- Charles Bloom
- Christopher Bortnem
- Nicole Burnham
- Bonnie Carlsen
- Cathy Corey
- Stephen Corey
- Ethan Debates
- Susan Debates
- Daniel Eaton
- Kristin Eaton
- Chris Harris
- Charles Huckabay
- Dione Kelly
- Michael Kiso
- Nicholas Lewis
- Priscilla McClaughry
- William McClaughry
- Cathy McMaster
- Gina Minyard
- Vekesh Mistry
- Stewart Monroe
- Kenneth Newman
- Jeffrey A. Nistler
- Minoo Nowak
- Brooke Ottman
- Molly Paulus
- William Paulus
- Lana Peth
- Lindsay Schaub
- Bruce Schoen
- Joan Schoen
- Sarah Seever
- Gwen Smith
- Sharon Stafford
- Ian Strand
- Anandi J Thompson
- Katrina Thurston
- James Villa
Washington
- Dale E. Carr
- Kelli Carr
- Gary L. Crawford
- Janet L. Crawford
- Kylee A Ferreira
- Jessica S Giron
- Yvette J. Lee
- Manuela Perkins
- Sandy F. Peterson
- Tracy L. Reuter
- Danielle M. VanWagner
- Loren R. VanWagner
RX Employees
California
- Brittany Ariza
- Andree Atanacio
- Shelema Atkins
- Mika Ballesteros
- Gloria Becerra
- Vandolf Boncato
- Andres Cao
- Miriam Cheng
- Susan Delmendo
- Laura Frutig
- Brent Guilliee
- Christine Guilliee
- Jonell Hutsell
- Alyssa Jewell
- Melanie Laforteza
- Alicia Lee
- Junior Lee
- Angelique Lopez
- Maricris Monroy
- Dev Naik
- Tu Kim Nguyen
- Roxanne Novak
- Jacqueline Nubla
- Karen Quintana
- Joey Ramos
- Lynnette Renfer
- Angeline Rinck
- Shy Rio
- Brenda Seffens
- Jeremy Supetran
- Alexis Tavizon
- Cierra Taylor
- Jimmy Tran
- Dustin Traver
- Raechel Urbano
- Paola Valencia
- Karla Vasquez
- Christine Wang
- Shi Zhong
- Kevin Zuniga
Nevada
- Ruben Baray
- Christopher Faine
- Mercedes Kerr
- Christine Pak
- Kimberly Rodgers
- Chanyis Taylor
- Ashley Terrell
- Eva Tomaszewski
Oregon
- Jamie Arnspiger
- Jamie Bahner
- Kyle Beechly
- Elaine Bent
- Rebecca Bertam
- Beth Biggs
- Frederique Blouin
- Lorene Boyd
- Rachael Boyd
- Cole Braun
- Savannah Braun
- Amy Braun
- Abel Bravo
- Elizabeth Bronson
- Emma Brown
- Susan Broxon
- Andy Bui
- Derrick Bulseco
- Christine Burg
- Douglass Burke
- Daniel Byers
- Sheryl Byrd
- Sergio Calvillo-Ruelas
- Eliza Capuia
- Bradley Chase
- Melissa Chase
- Steven Cheung
- Sherry Ching
- Emily Crouch
- Mirna Cruz Rodriguez
- Chante Cummins
- Leah Cummins
- William Davids
- Tamara Davis
- Kelcey Delach
- Brittany Denson
- Victoria Dubintsov
- Christian DuJordan
- James Emmons
- Leslie Esparza
- Scott Everett
- Terri Fagan
- Lorna Fell
- Debra Fergerson
- Alyson Ferrer
- Cassandra Frutos
- David Gambill
- Tammie Garcia
- Amanda Gaylord
- Samuel Gemeda
- Zeinab Goudiaby
- Nathan Hedin
- Margaret Hendrickson
- Erin Hewitt
- Jodiee Hickok
- Daniel Hockett
- Deborah Hull
- Aubree Humphreys
- Mark Hutchinson
- Ayelen Hyun
- Teresa Ide
- Ashley Johnson
- Heather Johnson
- Jhquetta Johnson
- Aleksandr Kaletin
- Alina Kaletin
- Shawn Knoetzel
- Zachary Korstian
- Irum Kotaiche
- Sophia Kwon
- Tami Laflen
- Lisa Lam
- Kenia Lamar
- Khoa Le
- Eric Lintner
- Mandy Liu
- Kyle Lopez
- Brittany Louie
- Sarah Love
- Maria Francia Marasigan
- Neil Marshall
- Carrie McClaughry
- Stephanie McPherson
- Judith McWhirter
- Jennifer Minter
- Mikayla Minyard
- Scott Mitchell
- Mark Moody
- Julia Moody-Warren
- Jimena Moreno Alvarez
- Kathy Morsett
- Nivarni Nand
- Dumitru Negreanu
- Emma Nesmith
- Mavrick Nesmith
- Richard Nesmith
- Lucas Newman
- Kevin Ngo
- Alicia Nguyen
- Chau Nguyen
- Hong Nguyen
- Thuy Nguyen
- Sandra O'Regan
- Alla Onishchenko
- Connie Ortega
- Leigh Overton
- Katie Penkert
- Jennifer Pham
- Kristie Phan
- Mona Phan
- Tina Pick
- Kimberly Pitts
- Glendy Quintanilla-Garay
- Adam Rachal
- Alicia Rachal
- David Rachal
- Teodora Raducheva
- Devin Rago-Vanderwall
- George Reed Jr.
- Alex Rivelli-Keagbine
- Robert Rizer
- Lia Robichaud
- Lorna Sager
- Michelle San Pedro
- Suzanna Schertz
- Makila Schuck
- Daniel Shively
- Howard Snyder
- Maricar Snyder
- Jessica Steinbrecher
- Lynn Strand
- Phillip Szeto
- Kathryn Tamburino
- Henry Tang
- Wayne Tayler
- Jennifer Tayler
- Kaitlin Taylor
- Taylor Thom
- Liana Tschen
- Timothy Unger
- Genevieve Van Herk
- Jennifer Vaughn
- Michael Warren
- Justine Weaver
- Madeline Welch
- Olivia Welch
- Laneah White
- Cassidy Williams
- Kacey Winchester
- Matthew Winters
- Edward Wisniewski
- Jonah Wright
- Hannah Wu
- Steve Xiong
- Tina Yarnell
- Tracy Yeamans
- Hyun Yi
- Cindy Zahrte
- Lacey Zimmerman
Washington
- Aleksandr Agusevich
- Nadezhda Agusevich
- Lindsay A. Binder
- Sefanit A. Birku
- Jannis A. Briones
- Danielle M Briones
- Catalina Bunescu
- Alexandra Castro
- Terika Chand
- Leanne DeJong
- Teri Ferreira
- Laura G Finney
- Juliana F. Fuentes
- Glenn D Galinato
- Christopher R. Giles
- Farishta Hashimi
- Theressa M Hecht
- Wei-Chun Hsia
- Andrea E. Huckabay
- Karissa M Ickes
- Heather L. Inczaukis
- Victoria Paige Kramer
- Rinku Lakhani
- Jim Lampkins
- Ria Y Landon
- Susan L. Lienhard
- Leilani Logan
- Uzoma O. Mbogu
- Joan McIntosh
- Cathrine R. Morse
- Julie A. Ngo
- Hop H Nguyen
- Christopher Nomura
- Max H. Oberholtzer
- Truong Pham
- Sandy Pheap
- Madina Popal
- Marwa Popal
- Eric Shane Reed
- Margarita Ruteva
- Kayla N. Sanchez
- John S. Sexton
- Shelly J Shim
- Hayden J Singh
- Sukhpreet Singh
- Karen Stuessi
- Marvin Hearns Talens
- Paul Tran
- Julie T. Truong
- Evgenia Volenko
- Holly D. Wallace
- John Wallheimer Jr
- Tracy Wisniewski
- Gloria Young
Vaccine Administrators
- Sherene Abat
- Bethany Aberle
- Nelly Ablog
- Bashir Abumenjel
- Luisa Alcantara
- Joan Alfano
- Alexandra Alverez- Padilla
- Fe Anderson
- Marjorie Andrews
- Kimberly Andrews
- Sarah Andrews
- Rebecca Araya
- Mayra Arce Vargas
- Divinity Arciaga Tanke
- Diana Armouch
- Denise Arthur
- Tom Ascher
- Christine Ashton
- Beverly Aydelotte
- Josepf Baccam
- Anna Bah
- sheryl Baker
- Rachel Balboni
- Fiori Bariagbir
- Erin Barnes
- Kathryn Baur
- Andrea Bennett
- Vanessa Benning
- Anthony Benthin
- Wendy Berg
- Anna Berka
- Bethany Berndt
- Steven Berning
- Mary Bethea
- Hailemichael Bezabeh
- Rebecca Binder
- Kimberley Birkemeier
- Jordan Blackwell
- Lyndsi Bobst
- Amy Bohna
- Christy Bomberry
- Creecian Boyce
- Sarah Bracken
- Jeannie Braden
- Leanne Brantley
- Michelle Braun
- Tamara Brown
- Brittany Brown
- Paul Browning
- Scott Buck
- Amanda Burdine
- Joe Byrne
- Traci Caddy
- Loreen Cadero
- Garrett Callahan
- Nicholas Callicoat
- Nicholas Callicoat
- Justin Cambridge
- Maria Cameron
- MELYSSA Campbell
- Laura Campbell
- Sierra Canites
- Caitlyn Cardona
- Samantha Caribardi
- Maryanne Carlson
- Brandi Caro
- Brita Carpenter
- Tracy Carr
- Giovanni Carrasco Gomez
- Tosha Carter
- Jean Carver
- Teresa Case
- Katrina Castillo
- Chelsi Cate
- Janet Caudill
- Jeanine Cavagnaro
- Kristen Chapman
- Summer Chatham
- Anayeli Chavez Lara
- Anne Chege
- I-Chun Chen
- Philip Cheng
- Swapna Chennareddygari
- Julie Chestnut
- Kathleen Christensen
- Jill Clark
- Thomas Clark
- Kendra Clark
- Shen Clark
- Terry Coe
- Ivy Coenelius
- Krystal Cohn
- Matthew Cole
- Lynn Cole
- Jennifer Colet Castaneda
- Phyllis Commeree
- David Connolly
- Sarah Conroy
- Kim Corey
- Kimberly Corey
- Carol Cortez
- Suzanne Cothern
- Flor Craig
- Brenda Cunningham
- Lalaine Dahlin
- Athena Dalton
- Tracy Danna
- Swaran David
- Tracy Davis
- Denise Davis
- Daphany Davis
- Elizabeth Davis
- Julia Davis
- Caroline Davis
- Joan Dawson Werner
- Sofia De Asis
- Aubrey De La Rosa
- Christina De Paz
- Rachael De Souza
- Noel Del Nodalo
- David DeLaRosa
- KARA DELCURTO
- Robin DeLeon
- Sequin DeMar
- Traci DeMarco
- Lucinda Demee
- Charina DeMille
- Esther DeWald
- Jason Dexter
- Amanda Dexter
- Lalaine Dhalin
- Jennifer Diehl
- Michael Dillinger
- Margaret DiValentino
- Sarah Donohue
- Michelle Doty
- Jennifer Dougherty
- Sheila Dozier
- Jodi Dressler
- Jean Drevdahl- Orchard
- Vera Dudik
- Margie Dunham
- Jessica Dunn
- Laura Dunn
- Penelope Dunne
- Shelly Dupell
- Michelle DuPree
- Leona DuWors
- Katie Edick
- Nikki Edwards
- Gale Edwards
- Kathleen Elias
- Dana Emmatrice
- Hazel Enriquez
- Ward Erickson
- Janneth Escalente
- Solande Estrada
- Gil Estrada
- Angela Evans
- MaryAnn Ewing
- Karli Fahrlender
- Shana Fain
- Benjamin Fallah
- Judi Faris
- Arash Fathizadeh
- Rebeca Feliciano
- Christina Fenerty
- LeAnn Fenwick
- Kaitlyn Fernandez
- Jeannie Fessenden
- Jacqueline Fisher
- Dan Fitch
- Kathy Fitterling
- Ulrike Fleck
- Nancy Flowers
- Laura Foster
- Benjamin Fowler
- Jodi Fowlkes
- Lynn Fox
- Jonathan Free
- Nicole Freeman
- Shannon Freeman
- Lisa Frey
- Kelsey Fyfe
- Elaine Gabriel
- Bernice Galicia
- Madeleine Gallaher
- Whitney Gange
- Pamela Garcia
- Kristena Gardner
- Annette Garner
- Victoria Gawlowski
- Laurie George
- Endalkew Gesese
- Cynthia Gill
- Margaret Gillien
- Erin Gingrich
- Hiwot Girma
- Erin GLIME
- Amanda Goergen
- Sara Golsan
- Shirley Gomez
- Lauren Gomez-Morayta
- Olga Goncharenko
- Taylor Gonzales
- Kimberly Gonzales
- Melissa Gonzalez
- Stephanie Gosselin
- Kendra Gouge
- Meggan Gould
- Howard Graitzer
- Kari Gratz
- Delena Graves
- Debra Gray
- Todd Green
- Lindsey Greene
- Angelia Gregg
- Steven Griffis
- Hawni Grillone
- Danniell Guffey
- Barbara Gulden
- Diana Haggerty
- Semere Hailemariam
- Meghan Hales
- Shelllie Halfhill
- Dawn Hall
- Erin Hall
- Mary Hamilton
- Donna Hammar
- Brennon Hand
- Breanna Handley
- Heidi Haney
- Kathy Hansen
- Emily Hanson
- Kelly Hardin
- Laurie Harris
- Andrea Hart
- Sandra Harwood
- Mohamud Hassan
- Lisa Hauk
- Paula Hawkins
- Sharon Hayes
- Charity Hazzard
- Jailee Head
- Nicole Heenan
- Priscilla Heimsoth
- Jane Hermano
- Ketewa Hesslup
- Cathy Hill
- Zarchi Hlaing
- Kari Hocker
- Lorraine Hoffman
- Jenny Hokenson
- Shawntina Holaday
- Michelle Hollenback
- Angela Holomb
- Paula Hoover
- Mary-Grace Hopkins
- Sarah Horton
- Nathanael Howe
- Cheri Hubbard
- Rachel Huffman
- Mimi Hughes
- Alyssa Hunt
- Jeff Hunter
- Cher Huppunen
- Andre Hussian
- Abdulaziz Ibrahim
- Whitney Ice
- Patrice Ingrassia
- Rachel Inman
- Adriana Iuras
- Deborah Ives
- Karissa Jackson
- Melody Jackson
- Calisha Jackson
- Robin James
- Gerry Jarabelo
- Lorie Jarvis
- Carol Jeffers
- Josh Jenkins
- Chelsea Jennings
- Kristine Jensen
- Jessica Jensen
- Sabrina Jensen
- Brandy Jensen
- Barbara Jimenez
- Donna Johnson
- Arlee Johnson
- MaryAnn Johnson
- Christine Johnson-Sundby
- Jackie Johnston
- Karen Jolicoeur
- Allison Jones
- Kathleen Jones
- Regina Jones
- Julia Jones
- Julius Kariuki
- Lisa Kas
- Ahmed Kassa
- Nanandi Katti
- Suzana Kayan
- Hailemariam Kebede
- Ingrid Keenan
- Debra Keith
- Tammi Kellington
- Lucy Kelly
- Fredrick Kennaday
- Susan Kenney
- Lisa Kensil
- Laura Kiepert
- Janet Kiernan
- Dayna Kim
- Hyeongyu Kim
- Kristina Kimmel
- LaKima King
- Kirstin King
- Katarina Kirby
- Elizabeth Knaff
- Amber Knapp
- Elizabeth Knauff
- Debbie Knauss
- Tonya Koeppel
- Matthew Kohler
- Kathryn Kolonic
- Tanya Kracheva
- Kimberly Kuhnau
- Soniya Kunwar
- David Kutch
- Cherie Kyalu
- Melissa Lackey
- Christina Lambert
- Sushkara Lamichhane
- Naomi Land
- Micheal Larsen
- Haley Laughlin
- Valerie Launius
- Joseph Laureta
- Robin Lawrence
- Melissa Lee
- Katherann Lee
- Yoonseon Lee
- Christopher Lehman
- Kiyomi Lehman
- Ashley Lehman
- Christina Lemons
- Monique Lerno
- Patrick Lesich
- Sandra Leverette
- Megan Levine
- Laura Lindberg
- Summer LLoyd
- Allen Lluisma
- Bobbie Loomis
- Andrea Loveday
- Annika Lowrey
- Megan Lucas
- Marietta Luman
- Erin Lunde
- Michelle Lutz
- Billy Mabe
- Kristi Mace
- Julie Maclaughlan
- Heather Madden
- Amy Magner
- Sabrina Mahamoued
- Pirtpaul Malhi
- Abby Manibusan
- Hailey Marbach
- Princess Mariano
- Vega Marilyn
- Erica Marsh
- Karen Marshall
- Bonnie Marshall
- Erica Martin
- Maria Martinez
- Bobbi Marugg
- Michelle Mason
- Shantell Mason
- Joan Matthews
- Danielle Mayberry
- Cricket McCloud
- Molly McCollum
- Cynthia McDaniel
- Callie McGrath
- Jeffrey McKay
- Susan McKennon
- Khrisna McKinney
- Susanne McLaughlin
- Caroline McMullan
- Leah Meadows
- Victoria Mears
- Shivani Mehta
- Alexis Melideo
- Bev menjivar
- Kayla Meredith
- Ruth Merrill
- Alicia Meuser
- Camille Meyer
- Joyce Meyersick
- Victoria Mganga
- Stephanie Miaoulis
- Brandi Miller
- Ruth Miller
- Brenna Miller
- Ruth Miller
- Julie Miller
- Mary Miller
- Brigitte Miller Rocha
- Christiana Min
- Joanne Miracle
- Kevin Moore
- Michael Moore
- Bailey Morisak
- Bryan Morris
- Ken Mortland
- Dawn Moskowitz
- Mary Munyua
- Carrington Muriu
- Blair Murphy
- Rafael Murray
- Kathleen Myers
- Susan Myers
- Lisa Neal
- Tamara Nelson
- Karen Neuenfeldt
- Leeann Newell
- Rose Ngigi
- Hien Nguyen
- Erin Nicks Martin
- Leah Nirschel
- Qiera Nixon
- Reanna Nocon
- Vicki Nordby
- Farrah Nosrati
- Katelyn Nottingham
- Stella Nsadde
- Kerry O’Brien
- Deborah O’Neil
- Kandice O’toole Stinnette
- Kathie O’Dell
- Sarah Obujen
- Kayla Odenthal
- Jennifer Ogle
- Jacey Oglesbee
- Rosana Oller
- Barbara Olson
- Eloizza Ortiz
- Christopher Ortolani
- John Osborn
- Rochelle Ostrom
- Ted Otto
- Nilo Ouano
- Carolyn Owen
- Joanne Ozaki-Moore
- Larisa Palanchuk
- Wilson Panoy
- Kimberly Parris
- Patricia Paruch
- Nancy Paschall
- Nicole Pashek
- Lisa Patterson
- Summer Pearch
- Ann Pedack
- Dionetta Pengilly
- Tamara Perzhu
- Sheri Pfost
- Rebecca Pham
- Zachary Phan
- Megan Phelps
- Ashley Phelps
- Brenda Phikulchakorn
- Ramona Picard
- Janelle Pioquinto
- Allison Polonsky
- Debbie Porter
- Elizabeth Price
- Kendra Prier
- Terri Pryne
- Lisa Pujol
- Erika Pullen
- Amy Ramirez
- Beca Ramos-Ochs
- Paetra Randall
- Jason Rankin
- Debbie Rayburn
- Carol Reed
- Makayla Reese
- Emmalie Reese
- Patricia Regalado
- Kari Regas
- Bethany Renner
- Sarah Reser
- Alicia Reyes
- Tiffany Rhoads
- Katie Richins
- Donella Rieke
- Christine Rito
- Susan Ritty
- Margaret Roberts
- Joni Robertson
- Sarah Robins
- Jocelyn Robinson
- Stefanie Robles
- Mary Roelike
- Clover Rose
- Michael Rosencrance
- Abigail Roth
- Valerie Rowe
- Lisa Rudisel
- Michelle Rushton
- Amanda Sadler
- Salina Sahle
- Sara Sahlfeld
- Jamaila Salenga
- Donna Salmeron
- Janelle Salvetti-Vanacker
- Pamela Sanchez Heier
- Tara Sanders
- Krystal Sanfillippo
- Christina Saunders
- Deborah Sayler
- Corinne Schambers
- Cindy Scherba
- Sheila Schneider-Mullins
- Debra Schnieder
- Connie Schoen
- Ryan Schroeder
- Alexandra Schulte
- Karen Sechrest
- Sengthip Seneboutarath
- Mackenzie Serrano
- Stacey Sheets
- Alisa Sheets
- Michael Sherwood
- Mary Shields
- Melissa Shields
- Larysa Shirota
- Ryan Shults
- Qabana Sima
- Katie Simpson
- Olga Simpson
- Connie Singer
- Bethany Sink
- Stephanie Sirisithi
- Nancy Slaymaker
- Laurie Slye
- Katie Smith
- Alicha Smith
- Babara Smith
- Pearlene Smith
- Filipinas Smith
- Breyane Smith
- Nia Smith
- Cindy Soares
- Diana Soto
- Renee Speck
- Vivian Spitza
- Lena Spohn
- Terryn Spragg
- Shawna Stallcop
- Earlene Stanley
- Jordan Stanley
- Jessica Stanley
- Julie Stanley
- Leslie Star
- Valerie Starr
- Alina Staub
- Patricia Steele
- Chelsea Steele
- Patricia Steele
- Naomi Sterba
- Dane Stevenson
- Scott Stewart
- Camille Stickel
- Michelle Stinson
- Amy Stivers
- Lisabeth Stolp
- Kristan Stone
- Rachel Strand
- Heather Stratton
- Emily Striplin
- Amy Sugg
- Casey Sullivan
- Jessiah Supanich
- Katie Suppan
- Marcie Suppe
- Tenaye Surbaugh
- Mindy Sylvestre
- Kelly Taaffe
- Monique Taoras
- Jacelyn Tappert
- Tina Tappouni
- Maria Taskar
- Deborah Taylor
- Maegen Taylor
- Joan Taylor
- Tim Teel
- Sharon Thiel
- Franciene Thompson
- Suesan Thompson
- Alanna Thompson-Poore
- Emmalee Thornton
- Michelle Thrapp
- Mary Timmol
- Denise Tinkham
- Rebecca Tobias
- Cynthia Tran
- Nadine Tranquilla
- Kayla Trosper
- Eric Troyer
- Laura Trupp
- Jeffrey Tubiolo
- Jessica Turner
- Carolyn Turner
- Matt Uemoto
- Teresa Uhl
- Sarah Urias
- Montana Usselman
- Emma Utterback
- Kale Valley
- Holly Van Loo
- Marisa Van Skike
- Melissa Vang
- Ceelina Ventura
- Maria Villegas
- Jennifer Vinuya
- Cheryl Voigt
- Reann Voorhies
- Elizabeth Waiss
- Janet Wakefield
- Donald Walker
- Andrea Walker
- Loni Walling
- Roy Wang
- Brian Ward
- Xenabelle Warner
- William Watkins
- Hailey Watson
- Debbie Watson
- Diane Waunus
- Valerie Weaver
- Robert Webb
- Suzanne Webber
- Lisa Weige
- Eric Weir
- Linda Wenger
- Lindsey West
- Cassie Whalen
- Jessica Wharton
- Dawn Whipp Groberg
- Tashauna Whitaker
- Michele White
- David Wilken
- DuAnn Will
- Aaron Williams
- Aaron Williams
- Caitlin Williams
- Michele Williams
- Stephen Williams
- Steve Williams
- Krystal Willingham
- Anthony Wilson
- Judy Wilson
- Thomasine Wilson
- Pat Winkler
- Barbara Winpisinger
- Nicole Wolfs
- Savannah Wood
- Lesa Wooton
- Pati Wright
- April Wright
- Melissa Wright
- Fangxin Ye
- Ana Ympa
- Christine Young
- Ana Ypma
- Nelly Yuzbasheva
- Anna Zentner
- Lorraine Zumwalt
Customers
- Adams House Assisted Living
-
- Jeff Bright
- Advocate Care
-
- Ben Fowler
- Leah Pedigo
- Alder Ridge Senior Apartments
-
- Sylvia Rouse
- Apple Springs Senior Living
-
- Devan Cooper
- Kathy Johnson
- Mary Wear
- Applegate Place Assisted Living Community
-
- Darcy Hood
- Tammy Schmall
- Arbor Oaks Terrace Memory Care Residence
-
- Katrina Hollo
- Arbors Memory Care
-
- Lisa Erck
- Barb Heywood
- Ashley Manor - Lund Lane
-
- Debbie Howerton
- Ashley Pointe
-
- Jeff Hendrickson
- Aspen Ridge Memory Care
-
- Jake Golden
- Aspen Ridge Retirement Community
-
- Bryan Carnahan
- Jan Robinette
- Barnett Woods
-
- Charley Parker
- Bay Pointe Retirement Community
-
- Haley Fitzgerald
- Jody Gauthier
- Jessica Ho
- Jennifer Zeh
- Bayberry Commons Assisted Living & Memory Care
-
- Chantel Mitchell
- Bridgecreek Memory Care
-
- Jody Burton
- Brightway Memory Care at Laurel Parc
-
- Anca Daniels
- Howard Dunkley
- Jenny Patoc
- Brookside Place Assisted Living
-
- Charles Davenport
- Brookstone Alzheimer’s Special Care Center
-
- Brandy Khlystov
- Callahan Court Memory Care
-
- Kim Jordan
- Heather Reid
- Callahan Village Retirement and Assisted Living
-
- Becky Benzel
- Tammy Huntley
- Cascade Manor
-
- Angelio Davis
- Penny Dunne
- Trisha Wygant
- Brian Young
- Cedar Crest Alzheimer’s Special Care Center
-
- Patty Odenborg
- Cheney Care Center
-
- Keith Fauerso
- Clatsop Care Retirement Village
-
- Launa DeGiusti
- Channon Larson
- Clearwater Springs Assisted Living
-
- Chasiti Ingram
- Hailey Watson
- Columbia Basin Care
-
- Aubree Schreiner
- Jasen Tennison
- Leana Tennison
- Columbia Ridge Senior Living
-
- Lorraine Brannum
- Emily Taghon
- Country Side Living in Canby North
-
- Roselynn Rockwood
- Country Side Living in Canby South
-
- Michele Quinn
- Sheena Willis
- Country Side Living Redmond
-
- Kathy Dominguez
- Cari Langenbach
- Courtyard Fountains
-
- Mary Beisley
- Nichole Bosma
- Bonni Henderson
- Edmonds Landing Assisted Living
-
- Gale Browne
- Tony Wilson
- eliseo
-
- Agnes Toribio
- Evergreen Court
-
- Mark Mullen
- Joy Mwiruki
- Evergreen Memory Care
-
- Amber K. Frye
- Heather Martinez
- Evergreen Senior Living
-
- Debby Johannes
- Sarah Morales
- Lindsey Robbins
- Farmington Square Beaverton
-
- Adriene Lierheimer
- Farmington Square Eugene
-
- Theresa Curcio
- Matt Hackett
- Jill Roblin-Maher
- Farmington Square Gresham
-
- Kalina Bounphisay
- Perla Gonzales
- Jessica Saray
- Maline Souliyalaovong
- Farmington Square Salem
-
- Jessica Penland
- Sarah Shipley
- Farmington Square Tualatin
-
- Jennifer Chongway
- Stace Jarvis
- Tammy Smith-Martin
- June Sulffridge
- Tawnya Theodore
- Fieldstone Cornell Landing
-
- Lucie Flood
- Krista Kinzer
- Amy Sandoval
- Forest Grove Beehive
-
- Tara Davis
- Charity Jammeh
- Fountain Court Senior Living
-
- Diana Foster
- Katie Kemp
- Fred Lind Manor
-
- Mari Carlin
- Dave Foltz
- Mimi Kamara
- Friendship Health Center
-
- Maile Cobb
- Ruth Deitz
- Dane Jensen
- Erica Knepper
- Jessica Nosen
- Christy Schomas
- Kim Williams
- Garden Court Assisted Living
-
- Samantha Chavez
- Heinz Gehner
- Nicole Purcell
- Chelsea Wright
- Garden Court Retirement Community
-
- Marra Coteng
- Generations Assisted Living
-
- Becky Bearden
- Caelyn Krech
- Glenwood Place
-
- Ben Young
- Harmony Guest Home
-
- Lucy Gallarde
- Harvest Homes
-
- Josie Cole
- Lyndia Moyer
- Candy Rolon
- Dulce ‘Candy’ Rolon
- Michelle Westwood
- Hawks Ridge Assisted Living
-
- Ben Brandt
- Highgate at Vancouver
-
- Melanie Danelson
- Steve Lin
- Autumn Mathis
- Highgate at Wenatchee
-
- Jean Lehman
- Sarah Robbins
- Holladay Park Plaza
-
- Christina Douvris
- Cindy Nelson
- Susan Platte
- Katee Samuelson
- HomePlace Special Care at Burlington
-
- Juliann Berg
- Wendy Haggard-Martin
- Jennifer Moody
- HomePlace Special Care at Oak Harbor
-
- Mykel Husk
- Horizon Ridge
-
- Anthony Christensen
- Donna Gillan
- Rhett Jensen
- John Mastrocola
- Rebekah Miranda
- Troy Reese
- Rocky Tanner
- Josephine Caring Community
-
- Alisha Hanson
- George Friou
- Shannon Goins
- Teri Lindgren
- Terry Robertson
- Sheila Wright
- Junction City Retirement and Assisted Living
-
- Nicole Hampl
- Juniper House Assisted Living and Memory Care
-
- Michelle Legore
- Johnnie McQuilkin
- Juniper Springs Senior Living
-
- Colleen Belmontes
- Shannon Carlton
- Jen Miller
- Kinsington Place
-
- Mary Blandau
- Edwina McCarthy
- Kinsington at Redwood Park
-
- Alison Andrews
- La Conner Retirement Inn
-
- Linda Hall
- Christina James
- Elena Vrinceanu
- Laurel Parc at Bethany Village
-
- Cynthia Le Tran
- Macdonald Residence
-
- Linda Nilsen
- Rose Wilde
- Manor Terrace Care Suites at Rogue Valley Manor
-
- Jill Howard
- Melissa Preston
- Marine Courte Memory Care Community
-
- Katie Bower
- Marquis Autumn Hills
-
- Jennifer Geer
- Scott Schuyler
- Erika Walters
- Marquis Centennial
-
- Sharron Hill
- Amy Loveless
- Amy Stone
- Marquis Centennial Hills
-
- Jacob Atwood
- Quinn Kohler
- Marquis Forest Grove
-
- Kathleen Chabreck
- Jennifer Cook
- Shehani Fernando
- Amanda Sandstrom
- Suesan Thompson
- Evan Windsor
- Marquis Hope Village
-
- Amanda Ballard
- Tracy Berg
- Marci Bird
- Caroline Brown
- Pilo Cano
- Jessica Comerford
- Kim Dossey
- Craig Gingerich
- Adam Harney
- Courtney Hohensee
- Melissa Laurandeau
- Karen Marshall
- Rhoda Parreno
- Martin Ruybalid
- Rebecca Safronchik
- Jamie Swartout
- Debbie Taggart
- Marquis Marian Estates
-
- Jennifer Hoffer
- Katherine Olsen
- Marquis Mill Park
-
- Denise Black
- Julia Ignashov
- Alexandria Kearney
- Brianna Rosser
- Jennifer Smeltzer
- Victoria Summers
- Erica Velez
- Marquis Mt. Tabor
-
- April Olsen
- Madison Pacheco
- Lisa Slavik
- Marquis Newberg
-
- Joscelyn Cook
- Dawn Jackson
- Shaylee Nannery
- Novi Ridjab
- E. Jordan Stanley
- Robert Thomas
- Marquis Oregon City
-
- Barbara Balmer
- Jordan Turner
- Marquis Piedmont
-
- Nolan Bocksteigel
- Kristy Cardwell
- Lorena Curtis
- Katie Greene
- Eleanor Jimenez
- John Kraemer
- Marie Leos
- Joan Maca
- Andy Robottom
- Sonia Salais
- Brittany Spray
- Shania Vang
- Jan Worley-Blazek
- Marquis Plaza Regency
-
- Lisa Meyer
- Sandy Connolly
- Leilani Romero
- Marilyn Saguran
- Marquis Plum Ridge
-
- Leslie Jacobsen
- Vanessa Morrow
- Christine Prather
- Marquis Springfield
-
- Derrick Landis
- Gabby Shorey
- Lisa Stedman
- Marquis Tualatin
-
- John Adlesich
- Jordan Costanzo
- Kylie Evenhus
- Tara Manske
- Stephanie Swayne
- Marquis Vermont Hills
-
- Fawn Aten
- MaryAnn Lagazon
- Karen Olomua
- Marquis Wilsonville
-
- Jessica Berry
- Valerie Geurtze
- Patty Kleckner
- Stephanie Larsen
- Melisa McDonald
- Jamie Weinel
- Martha & Mary
-
- John Cortina
- Heather Dart
- Rebecca ‘Aggy’ Feliciano
- Lynnette Ladenberg
- Sally Peregrino
- Amanda Pryslak
- Amanda Svoboda
- Maryville
-
- Kathleen Parry
- Maryville Memory Care
-
- Mylene Cepeda
- Melissa Vitale
- McKillop Residence at Marian Estates
-
- Shauna Garza
- Judy Sherman
- Meadowbrook Place Assisted Living
-
- Suzanne Miller
- Isela Regalado
- Meadowlark Senior Living
-
- Samantha Fauvor
- Andrea Fitzgerald
- Melody Court Memory Care Residence
-
- Christeena Hanson
- Trevor Taylor
- Memory Support Center at Rogue Valley Manor
-
- Mary Hollinger
- Merrill Gardens at Green Valley Ranch
-
- Sara Padilla
- Christine Perez
- Linn Thome
- Mirabella Portland
-
- Tristie Choe
- Stephanie Cook
- Michael Crivellone
- Megan Huston
- Kendra Robinson
- Mirabella Seattle
-
- Kenneth Castillo
- Jesan Frazier
- Amy Heider
- Loni Held
- Brooke Kasten
- Emmanuel Mensah
- Kinga Nagy
- Kevin Stallo
- Tina Tran
- Laura Yusim
- Mission Healthcare
-
- David Feeney
- Eva Karanja
- Mission Healthcare at Bellevue
-
- Lisa Boe
- Tara Travers
- Mission Healthcare at Renton
-
- Anafrid Amiani
- Heather Bills
- Erica Enrico
- Dell Workman
- Monterey Court Alzheimer’s Care
-
- Amy Kerslake
- Dawn Sparks
- Morrow Heights Assisted Living
-
- Nicole Cruz
- Pam Reed
- Mt. Bachelor Assisted Living and Memory Care
-
- Mallory DaCosta
- Irene Hernandez
- Maggie Jeans
- Don Seaton
- Ulysses Vargas
- Jennifer Williams
- Neawanna By The Sea
-
- Denna Lounseury
- Shawna Weist
- Nehalem Valley Care Center
-
- Debra Padgett
- Martha Parker
- Normandy Park Senior Living
-
- Kylene Daligcon
- Lily Fogleman
- Northridge Senior Living
-
- Memory Dent
- Lois Payne
- Ocean Ridge Assisted Living
-
- Diane Mason
- Kathy Sisson
- Olympics West Senior Living Community
-
- Sherri Hatch
- Elicia Paquette
- Oregon Veterans’ Home - Lebanon
-
- Abraham Andrade
- Monica Claflin
- Heidi Dallas
- Kassy Ketchum
- Candy Mull
- Valerie Nored
- Kelly Odegaard
- Oregon Veterans’ Home - The Dalles
-
- Katherine Buckley
- Cheryl Maitlan
- Tara Pray
- Our House of Portland
-
- Crystal Barber
- Gwen Dunham
- Mary Rita Hurley
- Pacific Gardens Alzheimer’s Special Care Center
-
- Laurie Barber
- Amelia Bevier
- Pacific View Senior Living
-
- Leslie Davis
- Carol Reed
- Penny Stark
- Malia Zartm
- Parkshore
-
- Annika DiNovi
- Roger Moore
- Katie Salyers
- Parkview at CherryWood
-
- John Goodwin
- Nicole Livingstone
- Olivia Peneyra
- Elizabeth Reining
- Heather Suitor
- Wendi Taylor
- Parkview at Wheatland Village
-
- Katie Allessio
- Brayden Strode
- Parkview Senior Living
-
- Jenelle Anderson
- Jennifer Johnson
- Patriots Glen
-
- Julie Brewer
- Jordan Drew
- Hind Essalmi
- Jane Whitaker
- Patriots Landing
-
- Gidgette Chesley
- Pine Ridge Alzheimer’s Special Care Center
-
- Gay James
- Sam Wyant
- Pioneer Village
-
- Beondi Hewson
- Dora Howard
- Eileen Morrow
- Prairie House Assisted Living and Memory Care
-
- Harold Bailey
- Rick Kennaday
- Steven Mays
- Love Pearson
- Princeton Village Assisted Living
-
- Doug Rusch
- Rackleff Place
-
- Alex Vice
- Raleigh Hills Assisted Living
-
- Lynda Brown
- Wendy Lilly
- Emilyanne Miller
- Keika Ruttan
- Yesica Zaragoza
- Redwood Heights Assisted Living
-
- Brianne Fowler
- Redwood Terrace
-
- sheryl Aldstadt
- Jim Thompson
- Marlys Wood
- Regent Court Senior Living
-
- Sherri Frost
- Zeth Owens
- Jennifer Roberts
- River Terrace Memory Care
-
- Jeffrey Wellington
- RiverWest Senior Living
-
- Mary Eisele
- Debbie Remsen
- Marla Starcevich
- Robison Health & Rehabilitation Center
-
- Krista Mattox
- Polina Munblit
- Rogue Valley Manor
-
- Lisa Mandell
- Amy Stonehill
- Stan Solmonson
- Rosewood Memory Care
-
- Angela Hernandez
- Royal Columbian Retirement Inn
-
- Robert Ogilvie
- Saphire at Liberty Pointe
-
- Kyle Thornton
- Saphire at Woodway
-
- Denise Brisbin
- Sea View Senior Living Community
-
- Donna Buick
- Cristi Parada
- Kerri Staggs
- Skyline at First Hill
-
- Jim Bennett
- Carol Foltz
- Rita Manley
- Kevin McNamara
- Greg Melin
- Ryan Miller
- South Beach Manor Memory Care
-
- Jeff Frandsen
- Logan Pratt
- Tina Tittle
- SouthTowne Memory Care
-
- Torie Greenly
- Spring Meadows Assisted Living Community
-
- Ana Arenas
- Tiffany Padula
- Pam Sessions
- Andrea Singleton
- Nina Wendelschafer
- Stafford Healthcare at Ridgemont
-
- Erin Bartz
- Roger Joice
- Adrienne O’Leary
- Stephen’s Place
-
- Heather Stenberg
- Summer Wood Alzheimer’s Special Care Center
-
- Laurie Ahmann
- Lynnette Arntson
- Irene Hyde
- Sunnyside Meadows Memory Care
-
- Jessica Melberg
- Sweetbriar Villa
-
- Brandy Harris
- Ivy Lizsow
- Table Rock Memory Care Community
-
- Marilyn Lawson
- Darren Penquite
- Tacoma Lutheran Retirement Community
-
- Noel Hermann
- Kevin McFeely
- The Adriana Senior Apartments
-
- Nan Baus
- Jeanne O’Brien
- The Amber Assisted Living
-
- Wanda Harris
- Heather Medina
- The Aspens Living Center
-
- Ryan Dupuy
- The Bellingham at Orchard
-
- Leanne Giese
- Adam Parker
- The Gardens at Juanita Bay
-
- Monika Carrillo
- Errol Porter
- Lucy Robles
- The Landing a Senior Living Community
-
- Shirly Keller
- Summer Lybarger
- Mark McClellan
- Elisa Moore
- The Lodge in Sisters
-
- Lisa Fortin
- The Oaks at Lebanon
-
- Angie Kutsch
- The Royal Anne
-
- Kurt Hagardorn
- Anna Hubenya
- The Springs at Anna Maria
-
- Christine Adams
- Vitoria ‘Tori’ Balfour
- Brenda Connelly
- Kathryn Teach
- Meaghan Walkup
- The Springs at Carman Oaks
-
- Amy Brown
- Melissa Carnell
- Sarah Coco-Findley
- Curchelle Ensign
- Kayla Harris
- Anna Neudorfer
- Vikram Robert
- The Springs at Clackamas Woods
-
- Amy Dudley
- Krista Krebs
- Olivia Lockard
- Becah McNaughton
- The Springs at Greer Gardens
-
- Victor Barredo
- Stephanie Dennis
- Brandon Hart
- Emory Healey
- Bard Howe
- Lauren Lebien
- Ashley Marshall
- Ashley Schulze
- Shalyn Wiltshire
- The Springs at Lake Oswego
-
- Jackie Fisher
- Jeff Herinckx
- Linh Ho
- Candice Marcks
- George Wheeler
- The Springs at Mill Creek
-
- Heather Jarding
- Naomi Ma’ae
- Toni Sly
- Jayce Tappert
- The Springs at Sherwood
-
- Sabrina Anhaack
- Talia Rivera
- Sara Sievers
- The Springs at Tanasbourne
-
- Eric Christensen
- Libby Hutter
- Victor Lanna
- Taylor McCaulley
- Linda Stanich
- Greg Young
- The Springs at Veranda Park
-
- Austin Mines
- The Springs at Willowcreek
-
- Maria Cortez
- Eva Daliana
- Richard Salazar
- Laarni Small
- Rebecca Staggs
- The Springs at Wilsonville
-
- Caroline Aldan
- Sonia Marquez
- Tim Minks
- Bernard Palmer
- Lynn Reverman
- Rachelle Story
- Michele Swisher
- Stacy Zimmerman
- The Suites Assisted Living & Memory Care
-
- Jennifer Ambeau
- Jay Pettit
- Angel Robertson
- The Taft Home
-
- Tiffany Coleman
- Karen Shenefelt
- Randee Skeen
- The Terrace at Beverly Lake
-
- Cory Peganyee
- Janet Tellier
- The Village at Keizer Ridge
-
- Anna Palomar
- Nancy Steers-Crist
- Timber Pointe Senior Living
-
- Dena Harrigan
- Kryssey Ponce
- Tammy Tucker
- Tri-Cities Retirement Inn
-
- Laura Samuel
- Vashon Community Care
-
- Katherine Daves
- Wendy Kleppe
- Wes McKey
- Village at Valley View
-
- Melissa Harris
- Village Concepts of Auburn - Brannan Park
-
- Sherlyn Bessette
- Danie Monaghan
- Village Concepts of Bothell - Riverside East
-
- Katie Blachly
- Betsy Frankie
- Village Concepts of Burien - El Dorado West
-
- Nichole Smith
- Village Concepts of Chehalis - Woodland Village
-
- Jessica Grey
- Halsey Luciano
- Village Concepts of Enumclaw - High Point Village
-
- Angie Howells
- Village Concepts of Fairwood
-
- Lynda White Eagle
- Village Concepts of Gig Harbor - Sound Vista Village
-
- Denise Mecartea
- Village Concepts of Hoquiam - Channel Point Village
-
- Cherie Bies
- Maggie Birmingham
- Brandon Mullins
- Village Concepts of Issaquah - Spiritwood at Pine Lake
-
- John Oates
- Linda Overland-Smith
- Brenda Pajo
- Margarita Reyes
- Michelle Strazis
- Village Concepts of Milton - Mill Ridge Village
-
- Raminder Nijjar
- Village Concepts of Oak Harbor - Harbor Tower Village
-
- Jessica Burley
- Katie Jones
- Village Concepts of Port Angeles - Park View Villas
-
- Gladys Doty
- Marthe Fortman
- Village Concepts of Sedro-Woolley - Country Meadow Village
-
- Sandra Jensen
- Vineyard Heights Assisted Living
-
- Kimberly Basilin
- Janet Sederquist
- Washington Care Center
-
- Esther Densmore
- Wheatland Village
-
- Tamara Gordon
- Tom O'Donnell
- Willow Place
-
- Becky Hollis
- Willson House
-
- Tabby Buchholz
- Donna Tweed
- Windsong at Eola Hills
-
- Heather Golden
- Woodside Senior Living
-
- Sherry Hogan
- Woodway Senior Living
-
- Alyssa Henderson
- Katie James
Partners
- American Health Care Association
-
- Courtney Bishnoi
- Mark Parkinson
- American Society of Consultant Pharmacists
-
- Chad Worz
- Centers for Disease Control and Prevention
-
- Ruth Link-Gelles
- Department of Human Services
-
- Elisa Williams
- LeadingAge Oregon
-
- Ruth Gulyas
- LeadingAge Washington
-
- Deb Murphy
- Managed Health Care Association
-
- Diane Koontz
- Stacey Ness
- Russ Procopio
- Michelle Templin
- OmniTech
-
- Jeremy Warner and team
- Oregon Health Authority
-
- William Beck
- Rachel Currans-Henry
- Trevor Douglass
- Rex Larsen
- Ashley Marshall
- Mike McCormick
- Lance Pugh
- Jennifer Stalsworth
- Genevieve Sundet
- Oregon Healthcare Association
-
- Phil Bentley
- Linda Kirschbaum
- Rosie Ward
- Signature Home Health
-
- Cody Feakin
- Kenna Feakin
- Mary Kofstad
- Statim
-
- John DiFiore
- Washington Department of Health
-
- Kathy Bay
- Washington Healthcare Association
-
- Robin Dale
- Vicki McNealley
- Lauri St. Ours
- Jennifer Summers
Home Office
- John Baker
- Daniel Carney
- Daniel Cowart
- April Diaz
- Ella Eberwein
- Drew Fogg
- Phil Fogg
- Zach Fogg
- Autumn Han
- Rick Jackson
- Matthew Johnson
- Angie Latta
- Andrew Leeland
- Nick LeVee
- Scott Miller
- Tyler Rachal
- Gary Tetz
- Laurie Thomas
- Nick West
- Kelsey Whittaker
- Katy Zahrte





